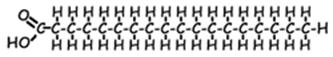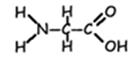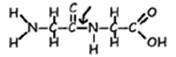Biochemistry study guide
Biochemistry study guide
The following texts are the property of their respective authors and we thank them for giving us the opportunity to share for free to students, teachers and users of the Web their texts will used only for illustrative educational and scientific purposes only.
All the information in our site are for educational uses.
The information of medicine and health contained in the site are of a general nature and purpose which is purely informative and for this reason may not replace in any case, the council of a doctor or a qualified entity legally to the profession.
Biochemistry study guide
Study Guide: Biochemistry
- Hydrophilic vs Hydrophobic. Since biological chemistry occurs largely in an aqueous environment, the interaction of a biological molecule with water is very important. That interaction is influenced by two primary causes: size and polarity (charge). The smaller a molecule is, the more likely it is to be willing to associate with water (dissolve). Also, the more polar and/or charged a molecule is, the more likely it is to be willing to associate with water. Since biological molecules are often very large, it is common for the different parts of the molecule to interact differently in water. For instance, a protein, which is composed of many different amino acids which have a large variety of characters, may be hydrophobic in part of its sequence and hydrophilic in other parts.
Hydrophilic (hydro=water; philios=love): Hydrophilic molecules or parts of molecules will dissolve in (interact with) water.
Hydrophobic (hydro=water; phobio=fear): Hydrophobic molecules or parts of molecules will refuse to interact with water. If sufficiently hydrophobic, a molecule or part of a molecule will actively repel or exclude water.
Hydrophilic/phobic characters are not an all-or-none phenomenon. Molecules fall along a scale, somewhere between extremely hydrophobic and extremely hydrophilic. Changing the parts of a molecule will often shift it more toward the hydrophobic or the hydrophilic end of the scale (depending upon the change).
- Hydrocarbons. The basic skeletons of organic molecules are composed of hydrocarbon. Hydrocarbon is made only of the elements carbon and hydrogen. Since carbon atoms all have the same electronegativity, and the electronegativity of hydrogen is only slightly different than that of carbon, the bonds in hydrocarbons are all non-polar. Thus, hydrocarbons tend to be hydrophobic, especially if they are more than a few carbons in size.
The many, many different organic molecules are formed by attaching a variety of functional groups to hydrocarbon skeletons. Each functional group has its own characteristic behavior, and the combinations of the behaviors of a molecule’s functional groups and the effects of the hydrocarbon skeleton create the overall nature of the molecule. Classes of organic molecules are largely characterized by their functional groups.
- Important Functional Groups
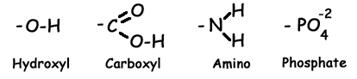
- Hydroxyl Group (found in alcohols and carbohydrates). This is a polar functional group due to the polar covalent bond between oxygen and hydrogen. Molecules whose most influential functional group is a hydroxyl group are called alcohols, and their systematic names end in –ol. Example: ethanol.
- Carboxyl Group (found in organic acids). The carboxyl is sometimes written –COOH. The H on the OH dissociates easily in appropriate circumstances, thus liberating H+ (a hydrogen ion) and creating –COO-. This makes this functional group a proton donor, and thus acidic. It also makes the carboxyl group a strong hydrophilic force. Molecules whose most influential functional group is a carboxyl group are considered to be organic acids; their systematic names end in –ate. Example: acetate.
- Amino Group. The amino is sometimes written –NH2. The amino group is polar, due to the polarity of the N-H bond. It also tends to pick up an extra hydrogen ion (due to the negative character or the N, and the pair of uninvolved valence electrons), thus becoming NH3+. This makes it a hydrogen acceptor, and thus alkaline in character. It also makes it a strong hydrophilic influence. Molecules whose most influential functional group is an amino group are organic bases, and their names end in –amine. Example: diphyenylamine.
- Phosphate Group: This functional group is always charged, either -1 or -2. This makes it strongly hydrophilic in nature and influence. Phosphates may be attached to organic molecules (organic phosphate) or unattached (free or inorganic phosphate). When diagramming the structures of phosphate-containing organic molecules, the organic phosphate is often abbreviated as a P inside a circle. Free phosphate is often abbreviated Pi (i for “inorganic).
- Structures of Important Classes of Biological Molecules
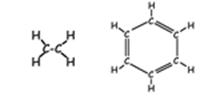
- Hydrocarbon: [hydro=hydrogen; carbon=carbon] These are molecules consisting of only hydrogen and carbon They come in many sizes and arrangements. They may be saturated (contain only single covalent bonds) or unsaturated (containing at least one double covalent bond). They are very non-olar, since the C-C bond and the C-H bond are both completely non-polar. Most carbohydrates are quite hydrophobic.
Examples: ethane benzene
|
|
|
|
- Carbohydrates: [carbo=hydrogen; hydrate-with water] Carbohydrates are molecules consisting only of carbon and the components of water (hydrogen and oxygen). In common language, most carbohydrates are sugars (saccharides) and their relatives. They fall into several categories.
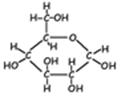
- Monosaccharides (mono=one). These molecules are composed of single sugar units. They come in a variety of sizes, depending upon the number of carbons in the sugar. The most common of the sizes is the hexose (hexa=six), which has six carbons. Also of biological importance is the pentose (penta-five), which has five carbons. There are several different hexoses and several different pentoses, and several may actually have the same molecular formula, since the details of the arrangement of the hydrogen and the hydroxyls on the ring is important in the identity of the sugar. Sugars are usually depicted in a ring form, though they also have open chain forms.
Examples: glucose (a hexose) ribose (a pentose)
|
|
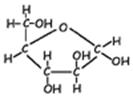
- Disaccharides [di=2]. These sugars are made of two monosaccharides covalently bonded together. The building of the linking bond is accomplished by dehydration synthesis (the removing of the components of water from the two sugars to be bonded together for the purpose of providing unpaired electrons for the formation of the new bond). Examples: sucrose (glucose-fructose) and maltose (glucose-glucose)
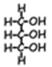
- Polysaccharides [poly=many]. These molecules consist of long chains (polymers) of monosaccharides linked together by covalent bonds (again, formed through dehydration synthesis). The long chains may branch. Examples: starch, glycogen, cellulose—all glucose polymers
- Lipids (fats and oils). These molecules are essentially all hydrophobic or partially hydrophobic. There is quite a variety of structure among the lipids, but the two best known examples are the steroids and the glycerides. The steroids include such substances as cholesterol, testosterone and estrogen. The glycerides include the triglycerides(primary storage lipids) and phospholipids (also called phosphoglycerides, the major component of all biological membranes).
- Steroids are complex structures with four interlocking rings. There are a number of them which have very significant biological roles. Your text can enlighten you on the structures and roles of the various steroids.
- Glycerides come in at least two types. All glycerides have as part of their structure the trialcohol glycerol, as well as two to three fatty acids (long hydrocarbon chains which terminate with carboxyl groups). The chemical nature of a glycerides is somewhat contradictory—especially the phospholipids. The glycerol part of the molecule is at least somewhat polar, while the hydrocarbon tails contributed by the fatty acids are highly non-polar. The fatty acids are attached to the glycerol through dehydration synthesis between the hydroxyls of the glycerol and the carboxyls of the fatty acids, creating an ester linkage. The formation of this connecting bond destroys most of the polarity of both the hydroxyl and the carboxyl. The completed glyceride has a polar head with two or three long non-polar tails.
Example: Glycerol Fatty Acid
|
|
- Triglycerides are constructed out of one glycerol and three fatty acids. The heads of these molecules have very little polarity left, and the tails are very non-polar. As a result, all triglycerides are at least mildly hydrophobic; most are very hydrophobic. The degree of hydrophobicity is determined by the lengths of the hydrocarbon tails from the fatty acids. The primary function of triglycerides is long-term carbon and energy storage.
Example: Triglyceride
|
- Phospholipids (phosphoglycerides) are very much like triglycerides, except that one of the fatty acids is replaced by a phosphate functional group. This affects the behavior of the molecule in two significant ways. First, it decreases the number of hydrophobic tails from three to two, thus weakening the overall force for hydrophobicity in the molecule. Second, since the phosphate functional group is small (and thus functionally part of the head of the molecule) and negatively charged, it is a very powerful force for hydrophilicity. Because of this arrangement, phospholipids spontaneously form bilayers in water, with the hydrophobic tails sequestered between the two layers of hydrophilic heads. The tails create a water-free environment between the two layers. This is the structural basis for all biological membranes. The membrane is a fluid mosaic composed of proteins of various sorts “floating” in a phospholipid bilayer.
Example: Phospholipid
|
|
- Proteins are long, unbranched chains (polymers) of smaller biological molecules called amino acids, linked together by a special covalent bond called a peptide bond. A peptide bond is a covalent bond between an amino group and a carboxyl group. Peptide bonds are formed by dehydration synthesis in a process called translation, which is performed by the ribosomes of the cell.
- Amino acids come in about twenty varieties. (Actually, there are a few more than twenty, and most come in two different stereoisomers, but since the translation process only specifies codes for twenty, those are the most significant in this context.) All of these amino acids have the same core structure—a carboxyl and an amino group with a single carbon between them—but each has a different R group (or side chain, sometimes called a “residue”) attached to that central carbon. R groups vary from a simple hydrogen (glycine) to complex ring structures (tryptophan). Some are polar, some non-polar; some are neutral, some acidic, some basic; some are small, some very bulky. As a typical protein might be between 150 and 500 amino acids long, this makes the possible variety in protein behavior very vast.
Example: Amino Acid
|
- Peptide bonds are the special covalent bonds responsible for linking the amino acids in a protein together. Since proteins vary in size, an average protein probably has between 150 and 500 peptide bonds. For this reason, proteins are often called polypeptides. NOTE that for a protein with quaternary structure (see below) the terms “protein” and “polypeptide” are not interchangeable.
Example: Peptide Bond
|
- Proteins have several layers of organization.
- Primary (1o) Structure: The primary structure of a protein is the simple amino acid sequence, held together by peptide bonds. The identity and behavior of a protein depends fundamentally upon what amino acids it contains and in what order they are arranged.
- Secondary (2o) Structure: The secondary structure of a protein consists of a variety of three dimensional configurations which have an organized, symmetrical appearance to the human eye. Secondary structure occurs as a result of the interactions of the polar bonds in the backbone of a protein, and are therefore due to hydrogen bonding. Not all proteins have secondary structure, and those which do have it vary in the percentage of the amino acid sequence which forms into secondary structure, depending upon a number of structural and sequence features in each protein. Two kinds are secondary structure are the alpha helix and the beta pleated sheet. Other kinds of molecules also have secondary structure. The famous double helix of DNA is that molecule’s secondary structure.
- Tertiary (3o) Structure: the tertiary structure of a polypeptide is its total three dimensional structure. It consists of a variety of folding, bending and twisting patterns, may of which make no obvious sense to the eye of a human being. However, all of the tertiary structure of a protein is important to its functioning, whether it seems to make sense to the observer or not. And for any particular kind of protein, the tertiary structure will be consistent from molecule to molecule of that protein. Since tertiary structure encompasses all of the three dimensional configuration of a protein, it includes the secondary structure. Overall, tertiary structure is produced by a variety of influences which occur between the R groups of the amino acids in the protein sequences. These influences include hydrogen bonding between polar R groups,
- Quaternary (4o) Structure: The quaternary structure of a protein involves the association between two or more polypeptide molecules, or between proteins and non-protein subunits (called cofactors or, if the protein happens to be an enzyme, coenzymes). Cofactors may be inorganic, such as metal ions (“minerals”) or organic, such as heme groups or vitamins. Not all proteins have quaternary structure. Only in the case of a protein with quaternary structure is the distinction between ‘protein’ and ‘polypeptide’ important. A protein is a complete, functional substance, with all of its parts included; a polypeptide is a single polymer of amino acids, with primary, secondary and tertiary structure.
Example of a protein with quaternary structure: Hemoglobin. Functional hemoglobin is composed of four polypeptide subunits covalently bonded together. Each of the subunits is covalently bonded to an organic cofactor called a heme group. A heme group is a porphyrin ring with a metallic cofactor—an iron ion (Fe+3). Hemoglobin, therefore, is an example of all types of quaternary structure.
Example of a protein with no quaternary structure: Insulin. Insulin is simply a single, rather short amino acid polymer, with no cofactors of any kind. It has 1o, 2o, and 3o structure. But no 4o structure.
- Nucleic Acids: There are two kinds of nucleic acids, deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). DNA is found largely in the nucleus of the cell, thus the general name, nucleic acid. (RNA is also found in the nucleus, though it is also present in the cytoplasm in large amounts.) Certain organelles also contain DNA (mitochondria and plastids). DNA and RNA are responsible for storing and implementing the genetic information in the cell. We will describe DNA, then characterize the differences between DNA and RNA.
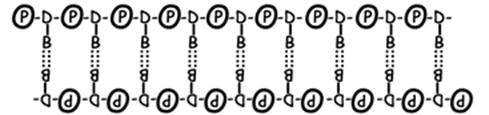
- DNA is a double polymer of smaller molecules called nucleotides. A nucleotide consists of one sugar (deoxyribose for DNA), a phosphate functional group, and one of four molecules called nitrogenous bases. The two nucleotide polymers are connected together via hydrogen bonding between complementary base pairs. Here is a schematic representation of DNA structure
|
In real space, the double polymer is twisted into a double helix
- The nucleotides in each polymer are covalently bonded together into a sugar-phosphate backbone. The nitrogenous bases are not involved in this backbone.
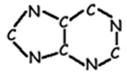
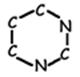
- In DNA, there are four different nitrogenous bases. (There is a fifth which is found only in RNA.) These bases come in two types, purines and pyrimidines. Purines consist of two interlocking carbon/nitrogen rings, and pyrimidines of a single carbon/nitrogen ring.
The purines are adenine (A) and guanine (G). Both are found in both DNA and RNA.
The pyrimidines are thymine (T) and cytosine (C). Cytosine is found in both DNA and RNA; thymine is found only in DNA. A third pyrimidine, Uracil (U) is found only in RNA.
Purine Skeleton |
Pyrimidine Skeleton |
|
|
- DNA has two sugar/phosphate backbones, with pairs of bases between them. (The size of a DNA molecule is usually described in terms of the number of base pairs in its length.) Each base pair has one purine and one pyrimidine, held in association by hydrogen bonding between the functional groups attached to the carbon/nitrogen skeletons diagrammed above.
- Because of the arrangements of function groups on the different bases, adenine will only pair with thymine (and vice versa) and guanine will only pair with cytosine (and vice versa). (In RNA, Uracil behaves just like thymine, so it base pairs with adenine.) In DNA, therefore, normally the only possible base pairs are A-T, T-A, G-C, and C-G.. The partners in these pairs of bases are described as complementary to each other. The other apparent possibilities do not occur unless there is an error in the replication of the DNA.
- This means that the base sequence of each side of the DNA molecule completely and accurately predicts the base sequence of the other side of the molecule (its complementary strand). This characteristic, complementary base pairing, is vital to the functions of DNA (and RNA). Complementary base pairing is responsible for everything that DNA and RNA do.
- The difference in the information contents of different DNA molecules (and therefore genes) is a function of the base pair sequence of the molecule. Since the length of a DNA molecule is undetermined (it can be almost anything), and the bases can come in virtually any order, there are literally an infinite number of different sequences possible for DNA, and, therefore, and infinite number of possible genes.
- RNA and DNA differ structurally as follows:
DNA |
RNA |
Sugar=deoxyribose |
Sugar=ribose |
Contains A, G, C, T |
Contains A, G, C, U |
Double stranded |
Single stranded (not helical) |
Very long |
Much shorter |
Source : http://www.cod.edu/people/faculty/fancher/Bio101/Biochem.doc
Web site link: http://www.cod.edu/people/faculty/fancher/Bio101
Google key word : Biochemistry study guide file type : doc
Author : not indicated on the source document of the above text
If you are the author of the text above and you not agree to share your knowledge for teaching, research, scholarship (for fair use as indicated in the United States copyrigh low) please send us an e-mail and we will remove your text quickly.
Biochemistry study guide
If you want to quickly find the pages about a particular topic as Biochemistry study guide use the following search engine:
Biochemistry study guide
Please visit our home page
Larapedia.com Terms of service and privacy page