Electrochemistry
Electrochemistry
The following texts are the property of their respective authors and we thank them for giving us the opportunity to share for free to students, teachers and users of the Web their texts will used only for illustrative educational and scientific purposes only.
The information of medicine and health contained in the site are of a general nature and purpose which is purely informative and for this reason may not replace in any case, the council of a doctor or a qualified entity legally to the profession.
![]()
Electrochemistry
Chemistry
Introduction
Your chemistry so far has focused on thermochemistry where so often a Bunsen burner has been applied to a reaction mixture in a test tube. Thermo means heat, hence exothermic for heat given out, as in a combustion reaction and endothermic which is a term applied to both physical and chemical changes where heat is taken in - as in boiling water for example.
Electrochemistry refers to electrical energy and because redox reactions often involve the transfer of electrons, there is a connection between these reactions and a flow of electrons - in other words, an electric current. This can be conveniently classified into two types of processes - and so -
There are two types of electrochemical cells.
Galvanic cells where a spontaneous redox reaction produces electrical energy.
Chemical energy is converted to electrical energy.
Electrolytic cells where electrical energy is used to force a non-spontaneous redox reaction.
Electrical energy is converted to chemical energy.
Both of these cells will be explained and related to some everyday situations - ranging from cells and batteries, to corrosion of metals, production and electroplating and refining of metals.
Galvanic cells
In a galvanic cell, also known as a voltaic cell, electricity is obtained from a chemical reaction. We use and rely upon, galvanic cells everyday. The torch battery, the car battery and the battery in your calculator contain galvanic cells. Although each of these batteries uses different chemical reactions, the principles of the way they work are the same.
In Medieval times, rulers employed charlatans posing as alchemists in the hope that they could convert a base metal such as lead into gold. These kings needed gold and lots of it to defend their kingdoms, to expand their kingdoms and to live an opulent lifestyle befitting their status. A convincing argument that a court alchemist was well on his way to success, was turning an iron nail into a copper nail simply by dipping it briefly into copper sulfate solution. Your teacher can demonstrate it to you. The iron nail is still an iron nail of course but coated with copper, giving the illusion that it is a copper nail. The reaction is:
Fe(s) + CuSO4(aq) → Cu(s) + FeSO4(aq)
Another example from the previous chapter is the reaction between zinc and copper sulfate. Both of these are electron transfer reactions - examples of redox. The equation for this reaction is:
Zn(s) + Cu2+(aq) → Zn2+(aq) + Cu(s)
The reaction occurs at the surface of the zinc. The electrons are transferred from the zinc to the copper ions as these strike the zinc. These are both metal displacement reactions and so familiar to you.
It is possible to set up apparatus which separates these two half reactions and makes the electrons flow through a wire from the zinc to the copper ions. Here is an example. It is called the Daniell cell.
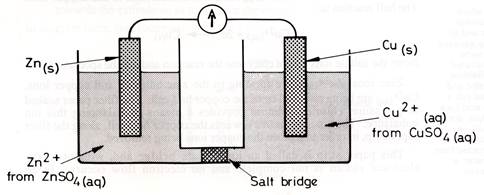
The chemistry is:
Zn(s) → Zn2+(aq) + 2e ─

through a wire
Cu2+(aq) + 2e ─ → Cu(s)
In galvanic cells, the separated compartments are called half-cells, and the reaction occurring in each half-cell is called a half-reaction. The zinc half-reaction is shown above. The electrons leave the zinc to travel to the copper half-cell via the metal wire connecting them. The electrons reaching the surface of the copper are captured by copper ions and they cover the surface with copper metal. The reaction in this half-cell is also shown above.
Note that the sulfate ions do not enter into this reaction and are the spectator ions. Zinc ions are forming in the zinc half-cell and copper ions are being removed from the copper half-cell.
The Daniell cell was the world's first commercial cell and is named after its inventor, John Daniell, who developed this while professor of Chemistry at King's College London in 1830. It was a glass or clay jar with a copper plate at the bottom covered with copper sulfate solution. A zinc plate suspended in zinc sulfate solution was in a separate, central, porous container. In the mid-19th century, Daniell cells were used mainly to operate telegraph networks but in some private homes they powered electric doorbells.
In laboratory galvanic cells, the porous barrier has been replaced by a salt bridge which connects the two half-cells and without it the circuit is not complete. The cell reactions will only occur when there is a complete circuit for the electrons to flow along.
Cell components
A circuit has two parts
● The external circuit where electrons flow through a metal wire
● The internal circuit where ions carry the current through the solutions in the salt bridge and half -
cells
The internal and external circuits are connected by the two electrodes.
● electrodes
The half-cell reactions occur here. The zinc and copper strips in their solutions are the electrodes in
your cell. Electrodes are made of metals or graphite - materials which conduct well in the solid state.
Graphite and platinum electrodes are unreactive and so are called inert.
● anode
By definition, the anode is the electrode where oxidation occurs. The more active metal zinc is the
anode in your cell because there is loss of electrons there. Anodes which are oxidized as the zinc is
here, are called active anodes. Active anodes are eaten away and get thinner.
● cathode
By definition, the cathode is where reduction occurs.
RED CAT will help you to remember this. The copper electrode in the above cell is the
cathode, because electrons are gained. Cathodes themselves do not react.
● electrolyte
This is a liquid containing mobile ions. There are three electrolytes in your cell - the zinc sulfate
solution, the copper sulfate solution and the potassium chloride solution connecting the two.
Electrolytes can conduct because they contain mobile ions. All aqueous salt solutions, molten salts,
aqueous acid and aqueous alkali solutions are electrolytes.
● Anions
These are negatively charged ions. They move towards the anode, hence their name.
● Cations
These are positively charged ions and move towards the cathode.
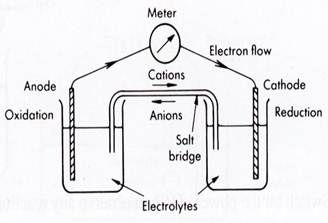
Using these terms, the operation of a galvanic cell can be described in sequence as:
● the loss of electrons at the anode - this is oxidation.
● These electrons move towards the cathode through the external wire. Reduction of cations in solution
occurs at the cathode.
● In the salt bridge, anions move towards the anode and cations move towards the cathode so as to
balance the circuit.
In diagram form this becomes:
Whenever two different metals are dipping into an electrolyte, a flow of electrons will occur from the more active metal to the less active metal when the circuit is connected. This can be demonstrated by dipping say zinc and copper electrodes into a potato or a grape or the same segment of a lime or lemon, which provide acidic electrolytes. A deflection of the needle on a voltmeter connected in the external circuit will be seen clearly. |
|
Have you ever heard the term galvanised into action? It describes someone who moves so suddenly, it is as if a sharp electric current has passed through, or they have been jabbed by an electric cattle prod. Luigi Galvani (1737 - 98) was an Italian anatomist who first recorded this. He was studying the effects of static electricity on the nerves and muscles of frogs. He was able to induce muscle spasms even if the legs were no longer attached to the frog and he believed he had discovered animal electricity. Alessandro Volta (1745-1837), a colleague and a physicist challenged this. He found that the effect was due to two dissimilar metals. Galvani's steel scalpel had touched the brass hook holding the frog's leg in place. The frog's body fluid would be the electrolyte. Galvanic cells are also called voltaic cells.
The sign convention in galvanic cells
When two dissimilar metals dipping into a solution of their ions are used, the combinations are referred to as galvanic couples. When the circuit is connected, electrons always flow from the more active metal to the less active metal. The more active metal, the anode, loses electrons more easily and so develops a higher negative potential and is given the negative sign. The cathode with a lower negative potential is positive, by default.
The salt bridge
The salt bridge contains a solution of potassium chloride, KCl and connects the internal circuit while keeping two different electrodes in the half cells from mixing. It also maintains electrical neutrality at the two electrodes. It answers the question: Why are anions which are negative, attracted to the negative anode and cations which are positive ions, attracted to a positive cathode?
Consider the half cell reactions in the Daniell cell.
At the anode (–) Zn(s) → Zn2+(aq) + 2e―
At the cathode (+) Cu2+(aq) + 2e― → Cu(s)
The overall reaction Cu2+(aq) + Zn(s) → Zn2+(aq) + Cu(s)
As zinc cations accumulate at the anode, outweighing the sulfate anions, there is a net positive charge accumulating at this electrode. These attract anions from the salt bridge. At the cathode, as cations are being reduced, coating the cathode with copper, a surplus of negative sulfate ions accumulate at this electrode. These attract cations from the salt bridge. Electrical neutrality is achieved and the cell chemistry can continue.
The ions in the salt used should not react with the ions in the solutions of the half-cells.
Galvanic cells - a summary
The following generalisations apply to galvanic cells where there are two different metals as electrodes.
● The more active metal will be oxidised.
● The ions of the less active metal will be reduced.
● Oxidation occurs at the anode (– vely charged electrode).
● Reduction occurs at the cathode (+vely charged electrode)
● Cathodes do not react.
● Electron flow through the wire is from anode to cathode.
● Cations in the salt bridge move towards the cathode.
● Anions in the salt bridge move towards the anode.
A cell diagram is a shorthand representation of the components of a cell, so for the Daniell cell, this becomes:
Zn(s)| Zn2+(aq)| | Cu2+(aq) | Cu(s)
where the single vertical lines represent phase boundaries and the parallel vertical lines, the salt bridge.
This shows two redox pairs with oxidation on the left hand side and reduction on the right.
Dry cells, miniature cells and batteries
Both cells and batteries are portable sources of electrical energy. Batteries are a group of galvanic cells connected in series, but the terms are often used interchangeably. This energy is produced by a spontaneous redox reaction, and in theory, any redox reaction can be used. However commercial cells are sealed units and stop working when the reactions are complete - in other words they go flat and are thrown away. These are called primary cells. Secondary cells - and the car battery is one, are rechargeable but to learn how this works, you have to learn first about electrolysis.
Electrolytic cells
Electrolytic cells use electrical energy to force a non-spontaneous redox reaction to occur. This process is called electrolysis. Electrolysis is used:
to produce reactive metals such as sodium, magnesium and aluminium,
to produce active non metals like oxygen, hydrogen, fluorine and chlorine and other useful chemicals,
to electroplate metals, to protect and beautify,
to purify metals. This is called electrorefining.
|
|
Factors determining the products of electrolysis
Electrolysis describes the process of decomposing a compound using an electric current.
The factors determining the products are:
1 The nature of the electrolyte
2 The concentration of the electrolyte
3 The nature of the anode - active or inert
4 The position of an element in the metal reactivity series.
Electrolysis of a molten salt
This is the method used for the production of metals at the top of the metal activity series - for metals described as anodic as they oxidise easily and form very stable salts which need a lot of energy to decompose. The diagram below shows the setup used for the electrolysis of a molten salt such as sodium chloride using inert electrodes. Molten sodium chloride would be the electrolyte.

The battery in the external circuit pushes electrons onto the cathode which in an electrolytic cell has negative polarity. The sodium cations, Na+ move to the cathode, attracted by opposite charge.
At the cathode (–) Na+ + e – → Na reduction
At the anode (+) 2Cl – → Cl2 + 2e – oxidation
Overall 2Na+ + 2Cl – → 2Na + Cl2
Electrolysis of molten oxides or halides gives oxygen or the halogen at the anode and the active metal at the cathode. Which metal is produced, depends upon which salt is used - of course!
Electrolysis of aqueous solutions
Electrolysis of aqueous solutions requires less energy than electrolysis of a molten salt as extra energy is needed to melt salts before electrolysis is possible. In the early 19th century, Sir Humphry Davy carried out electrolyses of the alkaline solutions of sodium, potassium and calcium hydroxides and was mystified when metals did not appear at the cathode. He could only get hydrogen at the cathode and oxygen at the anode. He then repeated his experiments by removing water and so was using molten alkalis. Thus in 1807 he isolated sodium and potassium, elements unknown until then and in 1808 he isolated calcium. He was the discoverer of all three elements. These appeared as silvery globules at the cathode, some of which ignited and burned with coloured flames. It is recorded that Davy danced with excitement to see these results.
Clearly it is easier to reduce water than Na+, K+ or Mg2+ - or the ions of any active metal.
The electrolysis of water produces oxygen at the anode and hydrogen at the cathode.
Oxidation of water at the anode (+) 2H2O → O2 + 4H+ + 4e –
Reduction of water at the cathode (–) 2H2O + 2e – → H2 + 2OH –
Pure water is not an electrolyte and dilute alkalis dilute acid or dilute sodium chloride and the ions in Adelaide tap water turn these into conducting aqueous solutions.
The electrolysis of concentrated sodium chloride
The electrolysis of dilute sodium chloride produces oxygen, not chlorine at the anode. The chloride ions are greatly outnumbered by water molecules!
However, the electrolysis of concentrated sodium chloride does give chlorine at the anode.
In the electrolysis on the right, using a Hofmann voltameter, universal indicator has been used.
Dark blue at the cathode shows the production of hydroxide ions. These turn universal indicator solution from green to dark blue.
2H2O + 2e – → H2 + 2OH – Red at the anode shows the production of hydrogen ions. These turn universal indicator solution from green to red. Chlorine produced at the anode makes the water acidic, but chlorine is also a bleach, so near the inert platinum electrode at the bottom of the cell the electrolyte is colourless.
2Cl – → Cl2 + 2e – |
|
The electrolysis of concentrated sodium chloride is carried out commercially to produce hydrogen, chlorine and sodium hydroxide. This is a high energy industrial preparation that consumes more energy than any other industrial preparation except aluminium.
Electroplating
Electroplating is is an example of the third point about electrolysis - that the reactions depend upon the nature of the electrodes. The object to be plated is made the cathode, and the plating metal the anode. The electrolyte contains ions of the plating metal. The chemistry of the chromium plating of a bumper bar, used on older cars, will make this clear. The cell is:

The cathode reaction is reduction Cr3+ (aq) + 3e – → Cr(s) plates onto the bar
The anode reaction is oxidation Cr(s) → Cr3+ (aq) + 3e –
The electrons are removed from the anode itself because it is easier to do so. The anode replaces the chromium ions Cr3+(aq), from the electrolyte which plates onto the bar.
If an inert anode was used, water would be oxidised, producing oxygen and hydrogen ions.
2H2O → O2 + 4H+ + 4e –
Electrorefining
Electrorefining means to make absolutely pure. Many metals are alloyed to improve their properties, but copper, one of the best metal conductors needs to be pure to conduct well. Impure copper, known as blister copper, is electrolysed at the anode to form greater than 99.99% pure copper at the anode.
The chemistry of the electrolytic refining of copper is similar to that of the plating process. The copper used in printed circuits, the basis for transistorised systems, e.g. calculators, radios and computers, and for general electrical work must be 100 percent pure. This high degree of purity is obtained by electrorefining.
The cell can be shown in diagram form as follows.
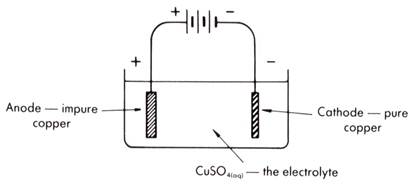
The chemistry is simple. At the anode the electrons are taken from the copper anions making up the anode and they are oxidised to copper ions which go into solution.
Cu(s) → Cu2+(aq) + 2e –
Silver and gold impurities are not oxidised, being less reactive than copper.
They drop to the bottom of the cell and form a sludge called anode mud. These precious metals are recovered.
Only copper ions react at the cathode, giving close to 100 per cent pure copper.
Cu2+(aq) + 2e – → Cu(s)
Electrorefining of gold occurs by a similar process. Impure gold anodes are suspended in an electrolyte of AuCl3 in hydrochloric acid. The cathodes are thin strips of pure gold. As oxidation proceeds at the anode, the anodes dissolve and the Au3+(aq) ions migrate to the cathode and are deposited there as 99.99% pure gold. Any platinum present in the impure anodes does not oxidise and sinks to the bottom of the cell. Silver impurities oxidise and precipitate as silver chloride - chemistry at work again!
Ag(s) → Ag+(aq) + e –
Ag+(aq) + Cl –(aq) → AgCl(s)
After several days, the solid gold cathodes are removed, melted, and cast into bars.
The similarities between galvanic and electrolytic cells
● Oxidation occurs at the anode.
● Active anodes get thinner.
● Reduction occurs at the cathode. Remember RED CAT!
● In the external circuit electrons conduct the current.
● In the external circuit, electrons flow from anode to cathode.
● In the internal circuit, ions in the electrolyte(s) conduct the current.
● In the internal circuit, anions move to the anode; cations move to the cathode.
● Anodes, if active, are eaten away, oxygen, iodine, i.e. non-metals and acid (H+ ions) are discharged,
● Cathodes do not react. Metals plate, hydroxide ions and hydrogen are discharged.
● When metals plate, cathodes get fatter.
The differences between galvanic and electrolytic cells
Galvanic |
Electrolytic |
● A spontaneous redox reaction produces electrical energy. ● Chemical potential energy is converted into electrical energy. ● There is no power source such as a battery in the external circuit. ● There may be a salt bridge in the internal circuit. ● There may be more than one electrolyte in the internal circuit. |
● A non-spontaneous redox reaction is forced by an external DC power source. ● Electrical energy is turned into chemical potential energy. ● There is a source of direct current, e.g. a battery in the external circuit. ● There is no salt bridge in an electrolytic cell.
● There is only one electrolyte.
|
Source : http://www.usc.adelaide.edu.au/local/transitionlectures/chemistry/electrochemistry.doc
Web site link: http://www.usc.adelaide.edu.au/
Google key word : Electrochemistry file type : doc
Author : not indicated on the source document of the above text
If you are the author of the text above and you not agree to share your knowledge for teaching, research, scholarship (for fair use as indicated in the United States copyrigh low) please send us an e-mail and we will remove your text quickly.
Electrochemistry
If you want to quickly find the pages about a particular topic as Electrochemistry use the following search engine:
Chemistry
Electrochemistry
Please visit our home page
Larapedia.com Terms of service and privacy page
