Thermochemistry heat and energy changes
Thermochemistry heat and energy changes
The following texts are the property of their respective authors and we thank them for giving us the opportunity to share for free to students, teachers and users of the Web their texts will used only for illustrative educational and scientific purposes only.
The information of medicine and health contained in the site are of a general nature and purpose which is purely informative and for this reason may not replace in any case, the council of a doctor or a qualified entity legally to the profession.
![]()
Thermochemistry heat and energy changes
Chemistry
THERMOCHEMISTRY
Thermochemistry Heat and Energy Changes
- Thermochemistry – the study of the energy changes that accompany physical or chemical changes in matter
- In the chemical reaction of the burning of ethyne:
2C2H2(g) + 5O2(g) ---à 4CO2 + 2H2O(g) + energy
the energy released is thermal energy, a form of kinetic energy,
resulting from the motion of molecules
- It is important to distinguish between the chemical system (substances undergoing a change) and the surroundings (the systems environment)
- The chemical system is the reaction itself while the surroundings include anything that could absorb the thermal energy e.g. air, containers, etc
- Exothermic reaction is one where the thermal energy flows into the surroundings from the system and an endothermic reaction is one where thermal energy flows into the system from the surroundings
- Thermal energy transfers as heat (q) between substances (remember an object can possess thermal energy but cannot possess heat)
- During a chemical reaction, chemical potential energy is converted into thermal energy which increases the temperature of the surroundings (e.g exothermic reaction)
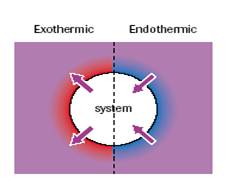
- An open system is one where both energy and matter can flow in or out of the system e.g. , most explosive reactions
- An isolated system is an ideal system in which neither matter nor energy can move in or out (theoretical)
- In reality we use a closed system where energy can move in or out but not matter (calorimeter)
Measuring Energy Changes: Calorimetry
- Calorimetry is the process of measuring energy changes in a chemical system
- Calorimetry depends on careful measurements of mass and temperature changes
- E.g. when a fuel (methane) burns it releases heat into the surroundings, if more heat is transferred, the observed temperature rise is greater
- i.e. a small amount of water will undergo a greater increase in temperature than a large amount of water (given the same amount of heat)
- mass (m), temperature (∆T), and type of substance are combined in an equation to represent the quantity of heat (q) transferred:

where: c = specific heat capacity (quantity of heat required to raise the temperature of a unit mass (e.g. one gram) by one degree Celsius or Kelvin (e.g. cH2O is 4.18J/(g x °C)
- quantity of heat (q) varies directly with the quantity of substance (mass, m), the specific capacity, c, and the change in temperature, ∆T

Sample Problem:
When 600mL of water in an electric kettle is heated from 20°C to 85°C to make a cup of tea, how much heat flows into the water?
Step 1: use density to find mass of water
m=DV
=1.00g/mL x 600mL
=600g
Step 2: use heat formula to calculate the quantity of heat transferred
where q = ?
m = 600g
c = 4.18J/g x °C
∆T = 85°C – 20°C = 65°C
q = mc∆T
= 600g x 4.18J x 65°C
(g x °C)
= 1.63 x 105J or 163kJ
therefore 163 kJ of heat flows into the water
Heat Transfer and Enthalpy Change
- chemical systems have various forms of both kinetic & potential energy
- kinetic energies include:
- moving electrons within atoms
- vibration of atoms connected by chemical bonds
- rotation & translation of molecules that are made up of these atoms
also include these potential energies:
- nuclear potential energy of protons & neutrons in atomic nuclei
- electronic potential energy of atoms connected by chemical bonds
- chemists can’t sum up all of these potential & kinetic energies of a system
- instead they study the enthalpy change or the energy absorbed from or released to the surroundings when a system changes from reactants to products
- enthalpy change or ∆H can be determined from the energy changes of the surroundings
- a useful assumption is that the enthalpy change of the system equals the quantity of heat flowing from the system to its surroundings or vice versa
- this assumption applies as long as no gas is being produced
- consistent with the law of conservation of energy – energy can be converted from one form to another, or transferred from one set of molecules to another, but the total energy of the system and its surroundings remains constant

e.g. Zn(s) + 2HCl(aq) ------àH2(g) + ZnCl2(aq)
- some chemical potential energy is converted into increased kinetic energy, then through collisions this energy is transferred to particles in the surroundings
- the enthalpy change is equal to the heat released to the surroundings
- we can observe this transfer of energy by measuring the increase in temperature of the surroundings
- to control variables and be able to make valid comparisons, energy changes are usually measured at standard conditions of temperature and pressure i.e. SATP, before and after the reaction
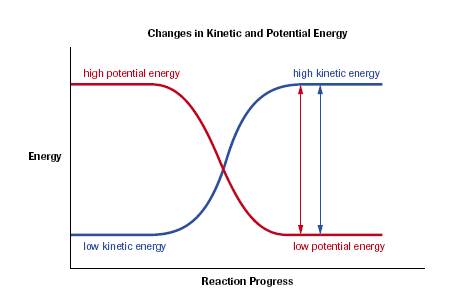
- in this case, the enthalpy change of a chemical system is the change in the chemical potential of the system because the kinetic energies of the system’s molecules stay constant (for our purposes now)
- we can observe enthalpy changes during changes of phase, chemical reactions, as well as, nuclear reactions.
- The magnitudes of the enthalpy changes may vary quite a bit but the basic concepts apply
Molar Enthaplies
- Consider the following thermochemical equation:

- The enthalpy change specified is per mole of H2 combusted and is therefore called the molar enthalpy of reaction - ∆Hr
- we can write this as ∆Hcomb = -241.8 kJ/mol
- We use the following sign convention:

- Molar enthalpies of reaction include the following:
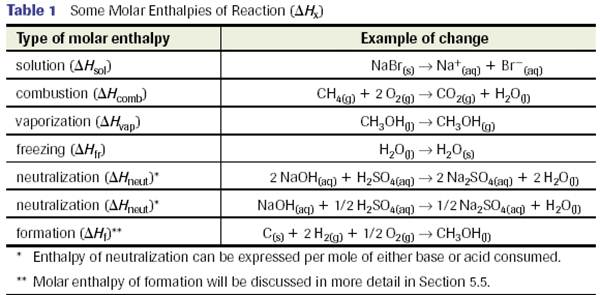
Molar enthalpies will generally be quoted in kJ/mole of substance of interest
Calorimetry: Physical and Chemical Changes
A calorimeter set-up appears below:

- Simplifying assumptions used in calorimetry are:
1. no heat is transferred between the calorimeter and the outside environment
2. any heat absorbed or released by the calorimeter materials, such as the container, is negligible
3. a dilute aqueous solution is assumed to have a density and specific heat capacity equal to that of pure water (D = 1.00 g/mL, c(H2O) = 4.18 J/g °C or 4.18 kJ/kG °C)
- Calorimetry measurements for physical changes include change of state, dissolving solids or change in temperature of solid, liquid or gas
- Calorimetry measurements for chemical changes usually involve aqueous reactant solutions that are considered to be equivalent to water
- eg. neutralization reactions – heat (or enthalpy) of neutralization
Representing Enthalpy Changes
Method 1: Thermochemical Equations with Energy Terms
Endothermic:
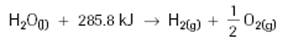
Exothermic:
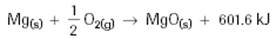
Method 2: Thermochemical Equations with ∆H Values
Endothermic:

Exothermic:

the units used for the enthalpy change are kilojoules (kJ), NOT kJ/mol, with the number of moles of reactants and products given in the equation
to state the molar enthalpy in terms of one of the reactants or products from the chemical equation one needs to consider the number moles of that substance in the chemical equation
Method 3: Molar Enthalpies of Reaction
- Standard molar enthalpy of reaction: the molar enthalpy that is determined when the initial and final conditions of the chemical system are at SATP (standard atmospheric temperature (25°C) and pressure (101.325 kPa))
- permit chemists to create tables to compare enthalpy values
- consider the exothermic reaction for the combustion of methanol

- the standard molar enthalpy is measured by taking into account all the energy required to change the reaction system from SATP to the reaction conditions to intiate the reaction, the energy released during the reaction itself, and all the energy released following the reaction as the products are cooled from reaction conditions to SATP
- the symbol used for molar enthalpy of reaction is ∆Hr to refer to reaction under consideration
Method 4: Potential Energy Diagrams
Energy changes can also be shown on chemical potential energy diagrams, where only the change in potential energy (∆H) of the system is shown in the diagrams

Hess’s Law of Additivity of Reaction Enthalpies
- How do chemists deal with chemical systems that cannot be analyzed using calorimetry?
- They use the principle that net (or overall) changes in some properties of a system are independent of the way the system changes from the initial state to the final state, i.e. the overall changes are independent of the path traveled
- The chemical potential energy of a chemical substance is dependent on its state or more specifically its temperature and pressure – it is NOT dependent on the way the substance is produced
- Many reactions can be explained by going through a number of intermediate steps
- In a chemical reaction the enthalpy change is dependent on the initial reactants and the final products, NOT the reaction steps that were followed to get the products

- Hess’s Law
The value of the ∆H for any reaction that can be written in steps equals the sum of the values of ∆H for each of the individual steps
Or
∆Hfinal equation = ∑ ∆Hknown equations
- the final equation has also been called the target equation, whereas the known equations refer to the reaction steps that are used to determine the final equation
Rules to follow in applying Hess’s Law
- if a chemical equation is reversed, then the sign of ∆H changes
- if the coefficients of a chemical equation are altered by multiplying or dividing by a constant factor, then the ∆H is altered in the same way

Multistep Energy Calculations
- We can now solve thermochemical problems using the different techniques developed thus far:
Heat Flow: q = m c ∆T
Enthalpy Changes: ∆H = n ∆Hr
Hess’s Law: ∆Hfinal equation = ∑ ∆Hknown equations
Standard Enthalpies of Formation
- Calorimetry and Hess’s Law are 2 ways of determining enthalpies of reaction
- A third way uses tabulated enthalpy changes (standard enthalpies of formation) for a special set of reactions called formation reactions, in which compounds are formed from their elements
i.e. 
Note kJ/mol are always stated for that quantity of substance.
Writing Formation Equations
- Always are written for 1 mole of a particular product, which may be in any state or form, but the reactant elements must be in their standard states
e.g. most metals are monatomic solids (Mg(s), Ca(s), Fe(s), etc), some nonmetals are diatomic gases (N2(g), O2(g), H2(g), etc) and the halogens show a variety of states (F2(g), Cl2(g), Br2(l), and I2(s))

See sample problem on page 331.
Homework: Nelson 12 Page 332, #1
Using Standard Enthalpies of Formation
- The equation for the formation of hydrogen gas is:
H2(g) ----à H2(g)
- The product and reactant are the same and so there is no change in enthalpy of this system
- We can generalize this to all elements in their standard states;

- Therefore enthalpies of Mg(s), Ca(s), Fe(s), etc are all zero
- We can use standard molar enthalpies of formation as a means of comparing the stabilities of substances e.g. graphite vs. diamond

- Graphite is the more stable form of carbon at SATP, so
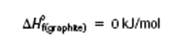
- Diamond is slightly less stable at SATP and has a greater potential energy than graphite (Fig. 1) and for the formation of diamond

See sample problems on pages 333 and 334
Note: Key Equation= 
Homework: Nelson 12 Page 335 #’s 2-5
Multistep Energy Calculations Using Standard Enthalpies of Formation
- Chemical engineers frequently need to do calculations to determine the heats produced by internal combustion engines
- Using problem solving skills we have learned to solve multistep
problems where enthalpy changes, heats transferred and masses of reactants were interrelated
- 2 key relationships we used were;

Source : http://www.leafrancis.net/Chapter5ThermochemistryNotes.doc
Web site link: http://www.leafrancis.net/
Google key word : Thermochemistry heat and energy changes file type : doc
Author : not indicated on the source document of the above text
If you are the author of the text above and you not agree to share your knowledge for teaching, research, scholarship (for fair use as indicated in the United States copyrigh low) please send us an e-mail and we will remove your text quickly.
Thermochemistry heat and energy changes
If you want to quickly find the pages about a particular topic as Thermochemistry heat and energy changes use the following search engine:
Chemistry
Thermochemistry heat and energy changes
Please visit our home page
Larapedia.com Terms of service and privacy page
