Organic chemistry
Organic chemistry
The following texts are the property of their respective authors and we thank them for giving us the opportunity to share for free to students, teachers and users of the Web their texts will used only for illustrative educational and scientific purposes only.
The information of medicine and health contained in the site are of a general nature and purpose which is purely informative and for this reason may not replace in any case, the council of a doctor or a qualified entity legally to the profession.
![]()
Organic chemistry
Chemistry
Organic Chemistry
The chemistry of carbon compounds is called organic chemistry. Why organic chemistry? The simple answer is that the first carbon compounds studied came from living organisms and because of this, were known as organic compounds. Today, organic chemistry is a separate branch of chemistry, because more compounds are formed from carbon than from any other element.
Approximately 90 per cent of all compounds are organic. The other main branch of chemistry is called inorganic - meaning non-organic chemistry.
The diagram below shows where carbon compounds are found in nature.
"The sun is the source that winds the clock of life."
Raymond B. Seymour
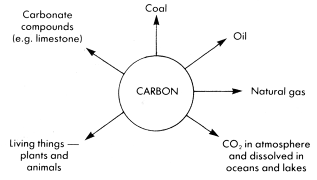
Until recently, it was thought that organic chemistry began when life began on this planet. Thus organic compounds were defined as those compounds obtained from plant and animal organisms, that is from living things. Furthermore, it was also believed that organic compounds could only be made in living tissue (in vivo) and not in test tubes in the laboratory (in vitro). This was called the vitalistic theory. As early as 1816 Chevreul, a French organic chemist, found that soap made from animal fat and alkali could be converted into other organic compounds. Twelve years later, Friedrich Wöhler, a German chemist, made the first organic compound from an inorganic compound when he made urea, a constituent of urine, from an ammonium compound.
After this, many organic compounds such as acetic acid were prepared in the laboratory. By 1850 the vitalistic theory had disappeared from scientific circles. The next step was to make carbon compounds not known in nature. These too are classified as organic compounds.
All the compounds in this diagram were originally formed from living things.
Methane, CH4, was believed to be part of t he eart h' s original atmosphere, together with water, hydrogen and ammonia, and as such could be considered to be the first organic compound.
In vivo, in life, from the Latin vivus, meaning living.
In vitro, in glass, from the Latin
vitrum, meaning glass.
The same principles that are used to explain inorganic chemistry, are used to explain organic chemistry. Chemistry today is unifiedSome people still argue that "natural" vitamin C for example
is superior to synthetic vitamin C even though they are identical.
A few carbon compounds such as carbon dioxide, metal carbonates and potassium cyanide are considered to be inorganic.
Where does the carbon come from?
Carbon dioxide from the atmosphere and water are the raw materials used by nature to make organic compounds. Plants are the factories and the sun provides the energy for the making of these compounds. Chlorophyll, a green pigment in plants, is the catalyst. This combination of carbon dioxide and water is an endothermic process and the reaction is called photosynthesis:
chlorophyll
6CO2 + 6H2O + solar energy 
glucose
The glucose produced is used by the plant to form other compounds, and it is from plants that animals get their required organic compounds in order to grow and move. Animals 'burn' organic compounds to provide the energy for movement and for maintaining body temperature. This burning of food can be shown by the equation:
6O2 + C6H12O6 → 6CO2 + 6H2O + energy
glucose
This reaction in animals is called respiration and is the reverse process to that for photosynthesis.
These reactions, photosynthesis and respiration, are summarised in what chemists call the carbon-oxygen cycle.
Properties of organic compounds
What do organic compounds have in common?
They all contain carbon.
They usually contain hydrogen as well, and often oxygen and nitrogen, and less often sulfur, phosphorus and chlorine.
They are all covalent compounds.
Because they are covalent compounds, they have the following properties:
|
• |
They are generally insoluble in water. |
|
• |
They have low boiling points and melting points - most organic compounds melt |
|
|
below 300°C. |
|
• |
They do not conduct heat or electricity. |
They almost all burn.
Organic compounds are used for fuels. Complete combustion of a fuel gives carbon dioxide, steam and lots of energy. Incomplete combustion gives carbon (soot) and carbon monoxide (CO). These equations show an incomplete and a complete combustion of butane.
C4H10 + 4O2 → CO2 + CO + 2C + 5H2O
2C4H10 + 13O2 → 8CO2 + 10H2O
You have seen these incomplete combustion products coming from the exhausts of inefficient car engines. Other products will form if other elements are present in the organic compound.
Organic reactions tend to proceed slowly.
Organic reactions require the breaking and then reforming of strong covalent bonds, and are usually slow. To speed up these slow organic reactions, heat and catalysts are used in the laboratory, while heat, catalysts and high pressure are used in industry. Extremely efficient biological catalysts, called enzymes, are used in living systems to bring about complex organic reactions at moderate temperatures.
But why are there so many carbon compounds?
Carbon as an element is fairly inert at room temperature; nevertheless it has the ability to combine with almost every other element in the periodic table. However, an enormous number of compounds is achieved with carbon combined with relatively few other elements. These other elements are hydrogen, oxygen, nitrogen, sulfur, phosphorus and chlorine.
There are a number of contributing factors that explain why carbon forms so many compounds. These are:
Carbon is tetravalent.
Tetravalent means to form four bonds. Carbon with its electron configuration of 2,4 is capable of sharing its outer shell electrons with four other atoms to form four new bonds. In doing this, it achieves the stability associated with an octet of electrons in its outermost shell. These four bonds are covalent.
Carbon can form single, double or triple bonds.
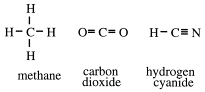
These are called valence bond structures. They show each covalent bond as a short straight line. This property is not unique to carbon because oxygen, for example, forms single and double bonds, and nitrogen can form single, double or triple bonds. But carbon has one property no other element has to the same extent, and that is its ability to form carbon chains.
Carbon can form covalent bonds with other carbon atoms to form chains of any length.
To form compounds, at least one other element must be present. Hydrogen is the most common. Compounds containing carbon and hydrogen only are called hydrocarbons. The simplest hydrocarbon is methane, CH4 .

This is methane. Remove one hydrogen and replace with a carbon. This carbon is tetravalent too. The remaining three links bond to hydrogen.

This is ethane, C2H6. Remove one hydrogen and replace with a carbon. Add hydrogen as before.
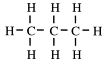
This is propane, C3H8. Remove one hydrogen and replace with a carbon atom.
Can you draw elect ron dot structures for these compounds?
Valence bond structures are also called graphic formulae.
Chemists often refer to CH4 as the simples t of t he organic compounds.
The ability of carbon to form long or short carbon chains is called catenation, from the Latin catena, meaning chain.

To do carbon chemistry you must be able to count up to four.
And swiftly as we see wines flow, yet sluggish olive oil will take its time undoubtedly because it is composed of atoms either longer or more hooked and mutually entangled.
Lucretius, De Rerum
Natura, about 57 BC
A.S. Couper and A. Kekulé were the first to propose that carbon is tetravalent and that it can link to form chains. Couper went further; he invented graphic formulae. In
1858, Couper asked Wurtz to present his ideas to the French Academy, but Wurtz failed to do so and t hus K ek ulé' s paper was published first.
Kekulé subsequently received most of the credit. Couper was bitterly disappointed and he never returned to the serious study of chemistry. Kekulé became one of the most famous organic chemists of all.
This is butane, C4H10.
This process can be repeated to form chains of any length. What you are actually adding when you extend the chain by one carbon atom is not just carbon but CH2!
Predicting physical properties.
The simplest hydrocarbon is methane. Natural gas is mainly methane. Hexane is a hydrocarbon with six carbon atoms. Your teacher will show you an example of hexane, C6H14. Your wash bottle is made from a king size hydrocarbon called polythene. The number of carbon atoms in a polythene molecule can be as high as 200 000!
What is the most obvious visual difference between these three hydrocarbons? Answer: Methane is a gas, hexane a liquid and polythene a solid. The longer the
chains are, the slower moving the molecules are. You would expect long, slow-moving
chains of carbon to be solids at room temperature and little light molecules to be gases.
Predicting chemical properties.
Notice that all these molecules have C_H bonds and, apart from methane, all have C_C bonds as well. You wo
uld expect the chemistry of methane, ethane, propane and butane to be similar. Their chemistry is similar and all of these hydrocarbons belong to the same chemical family.
Homologous series.
Organic chemistry is organised to make the study of this subject as easy as possible. There are far too many compounds to allow individual studies of each, so a system must be used. The system chemists use is to put carbon compounds into families. These families of compounds are called 'homologous series'. Homologous means the same. Each family of compounds is given a name. 'Alkane' is the family name for a group of compounds of carbon and hydrogen.
Homologous series have four characteristics:
1. Each member has similar chemical properties.
2. The physical properties change gradually with increasing chain length:
(a) The melting points and boiling points increase with increasing chain length. (b) Solubility in a given solvent decreases.
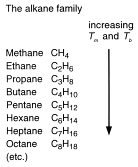
4. The mathematically minded of you may have realised that the formula of alkanes can be represented by a general formula. This is the final characteristic of a homologous series. Each homologous series can be characterised by a general formula.
The general formula of the alkanes is CnH2n+2 and they are said to be 'saturated'. The terms have nothing to do with being saturated with water. They contain the maximum amount of hydrogen - unlike other hydrocarbon homologous series. They are 'saturated' with hydrogen.
Saturated hydrocarbons contain only C_H and C_C bonds. There are no carbon- carbon double bonds; there are no carbon-carbon triple bonds. Compounds with 
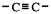
Arrangement of the atoms.
Chemists call the arrangement of atoms the structure. The structure of a molecule refers to the arrangement in space of its atoms. Whenever carbon is singly bonded to four other atoms, the carbon is at the centre of a tetrahedron. Methane, for example, is a tetrahedral molecule; so is carbon tetrachloride.
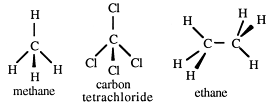
Ethane can be seen as two linked tetrahedra. Can you visualise what happens in chains? A continuous carbon chain is referred to as a straight chain and, for convenience, it is usually drawn that way.
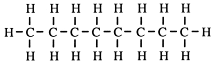
However, using models, which have the correct tetrahedral bond angle (109.5°), it can be seen that strictly the chain is a zigzag one - or kinked or puckered because of the tetrahedral bond angle. Your teacher will show you this.

But some of the carbon atoms can form branches on this chain. Here is an example of a branched chain alkane.

More about these later.
Formulae
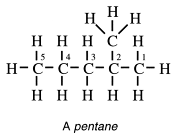
Two of the representations above are examples of graphic structural formulae or valence bond formulae of straight-chain compounds. Notice that at the ends of a straight chain each carbon atom is joined to three hydrogen atoms. Those in the middle are joined to two hydrogen atoms. We can write structural formulae in a simpler way. For example, pentane could be written:
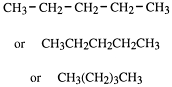
and these are called condensed structural formulae.
The arrangement of bonded carbon atoms is referred to as the carbon 'skeleton'; the hydrogens are thus like a 'skin'.
Can you remember what an empirical formula is?
In the middle of the 19th century there was great confusion among chemists about the ways of writing organic formulae - for example there were 19 different versions of the formula of acetic acid, which has only eight atoms!
There is geometry as well as arithmetic to a molecule.
Structural isomers are compounds with the same molecular formulae but different structural formulae. What, then is an isotope?
Of the top 20 companies in the world today, seven are oil companies.
An empirical formula is the simplest whole number ratio of atoms in a molecule. For methane, this is CH4. What is the empirical formula for ethane?
The molecular formula of a compound is the actual number and kind of atoms present in a molecule. Methane's molecular and empirical formulae are the same. The molecular formula of ethane is C2H6.
Structural formulae give some idea of the arrangement of the atoms in space, e.g.
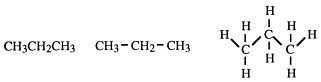
are all examples of structural formulae. They show which atoms are joined to which.
Graphic formulae will be easier for you to write because they show at a glance that each carbon has four bonds around it. Carbon must have four bonds, no more and no less, because four electron pairs are shared to complete its outer electron configuration.
Why use a structural formula instead of a molecular formula? The answer is that one molecular formula may apply to two different organic compounds - or to twenty or to over two million. A structural formula specifies which compound is being referred to.
Structural isomers
Compounds with the same molecular formula, but with different chemical structure, are called structural isomers. Isomers are different chemical compounds. As simple a molecular formula as C2H6O is found to represent two quite different compounds. Here is one, ethanol:

Remembering that carbon has four covalent bonds, oxygen two, and hydrogen one show the other way these atoms can be joined. Your teacher will help you here. The other compound is called dimethyl ether.
The organic chemistry industry
We use organic compounds for many things. They produce:
• the chemicals we use, such as medicinals, dyes, polymers, plastics, solvents and
detergents;
• the fuel we seem to take for granted.
The same organic compounds are used to provide both.
The organic chemicals used in industry come from fossil fuels. These are the remains of dead plants and animals reduced to their most stable form, that is they are generally a saturated hydrocarbon mixture, though another stable hydrocarbon family is also represented. These fossil fuels are:
• Natural gas - this is mainly methane, plus small amounts of ethane, propane and butane.

natural gas and petroleum.
• Coal - this has been an important fuel, and could become important again if petroleum sources dry up. The chemicals made from coal are mostly those we cannot get easily from other sources.
Hydrocarbon humans
The twentieth century is so dominated by oil in national strategies, global politics and power, it is hard to believe that no one thought it might be useful on a large scale until
1854 when Professor Silliman of Yale University, USA was engaged to report on the properties of Pennsylvania rock oil as an illuminant and lubricant.
In its first decade, petrol was a useless by-product of kerosene and was often run out into the rivers at night. Kerosene was known as "the new light". Kerosene lamps pushed back the night and extended the working day.
By the end of the 1870s, the Standard Oil Company, owned and run by John Rockefeller, dominated world trade in kerosene. 'Petroleum,' said Standard Oil 'has forced its way into more nooks and corners of civilised and uncivilised countries than any other product in business history.'
The first foreign competition came from the Nobel family. Robert Nobel bought an oil refinery in Baku, an outpost of Russia, with brother Ludwig's money.
Ludwig Nobel expanded the business and became known as 'The oil king of Baku.' Ludwig invented the first oil tankers - little more than floating bottles - and built a crucial 42 mile long pipeline ahead of competitors by blasting through a mountain with 400 tons of dynamite supplied by his even more famous brother Alfred Nobel.
The emphasis on kerosene's importance diminished when the chief engineer working for Thomas Alva Edison's illuminating company in Detroit decided to branch out on his own to design and sell a petrol-powered car, named after him. This was the model T-Ford.
At the beginning of the twenty first century oil still dominates security, prosperity and the nature of civilisation. It provides most of the energy and much of the chemicals used to run our society, which today is truly a "hydrocarbon society."


We use petroleum products at a rate conservatively estimated to be about one million times faster than their rate of formation.
"Oil - the essence of old sunshine."
Sir John Cornforth

Petroleum deposit
LPG stands for liquid petroleum gas. It is used as a camping gas and for portable gas supplies.
The oil refinery
Fractional distillation
A refinery separates crude oil by distillation into fractions, which are collections of hydrocarbons of different chain length. The process is called fractional distillation; a separation into parts or fractions. Refineries are complex operations, which are controlled by computers. South Australia's oil refinery was at Port Stanvac.
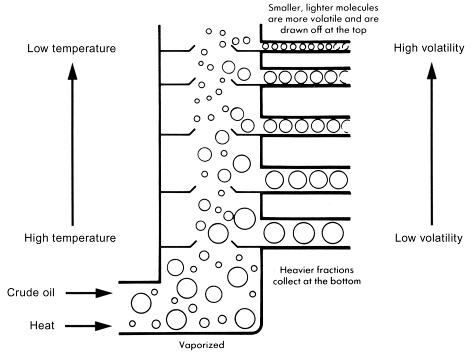

All these fractions become progressively thicker, more viscous and more difficult to ignite.
Octane number
The quality of petrol is measured by its octane number. In an internal combustion engine, like that of the modern car, a mixture of air and vaporised hydrocarbons in the C4-C12 range (petrol) is drawn into the cylinder on the down stroke of the piston. This mixture is compressed by the piston on its upstroke and then ignited by a spark from the spark plug. A high-octane petrol will burn quickly and smoothly giving a surge of power and forcing the piston down into its next stroke. A low-octane petrol, on the other hand, will explode, setting up shock waves that batter the piston and cylinder walls, giving a knocking sound.
A good petrol contains branched hydrocarbons. An example is:

This is called isooctane, or 2,2,4-trimethylpentane. You will learn how to handle these names with confidence later in this chapter. Isooctane has an octane rating of 100.
A 'poor' petrol is heptane. This is the graphic formula for heptane:

There are no branches to this chain of carbon atoms. This petrol has been given an octane rating of zero.
Other fuels are assigned octane numbers by comparison with these two - for example petrol with an octane number of 75 would show the same burning characteristics as a mixture of 75 per cent isooctane, 25 per cent heptane.
One method of improving octane rating is to use tetraethyl lead, but this results in poisonous lead compounds being emitted from the exhaust. Cheap lead-free, high- octane fuel relies upon the addition of other hydrocarbons produced by refinery reactions. These methods were developed in the 1930s but not adopted by the petroleum industry as a whole until the 1970s. More oil is now needed for fuel than before.
Refinery reactions
Physical separation is only one aspect of the function of an oil refinery. Another important role for the chemist is to change some of the less useful products into more useful ones.
Fuels, especially petrol, are in great demand and less useful fractions are converted into this fuel. The distillation of crude oil does not provide enough petrol, and it has a poor octane rating. Chemical technology is used to solve this problem - to create products with the properties we want them to have.
What sorts of alkanes are in crude oil? The answer is mostly straight-chain alkanes. The main problem for the chemist is that the branched-chained hydrocarbons are the better fuels. The more branched-chained molecules present, the better the petrol.
The octane number of leaded petrol is about 97, and unleaded petrol 92.
Knock increases engine wear and leads to wastage of petrol.
Octane number of some hydrocarbons
Pentane |
62 |
Hexane |
19 |
Heptane |
0 |
2-methylpropane |
122 |
2-methylbutane |
99 |
2-methylpentane |
83 |
3-methylpentane |
86 |
2-methylhexane |
41 |
3-methylhexane |
56 |
2,2-dimethylpentane |
87 |
2,3-dimethylpentane |
87 |
Benzene |
99 |
This is why most petrols contain up to about 30% benzene!
Crude petrol has an octane number of less than 60.
In 1921, after a three-year search, Thomas Midgley discovered that the poisonous, oil-soluble compound tetraethyl lead, Pb(CH2CH3)4 was a cheap, anti-knock compound. As a result, he became a very wealthy man.
Petrol is a volatile mixture of hydrocarbons, both cyclic and non
-cyclic, in the range of pentanes to decanes. The quality of petrol is very much dependent on the hydrocarbons of which it is composed. In the C4 - C12 range, there are 661 possible alkanes. Therefore, there is no single formula for petrol.
A catalytic cracker is an important installation in an oil refinery. The USA processes 12 x 106 barrels a year (a barrel is 284 L), and of this, one third is cat. cracked.
Certain rocks called zeolites are now being used increasingly as catalysts. These rocks have a suitable uniform pore size and catalytic action is on the inside surface. These catalysts are more selective in producing molecules of the right chain length, and are faster, as there is less coke build up.
Cracking is important for two reasons:
• It produces more widely used
compounds from less useful ones.
• It prod uces un sa turate d
hydrocarbons.
Do you notice anything unusual about any of these formulae?
Methods of converting other fractions into C4 - C12 hydrocarbons
1. Small alkanes are stuck together to make bigger alkanes - but the percentage of lighter alkanes is small and, as these alkanes are also in demand, this reaction is not used very much in practice.
2. Cat. cracking - 'cat.' here stands for a catalyst. Cracking larger molecules into smaller ones of suitable size was first achieved using high temperatures alone, but with suitable catalysts (a type of clay containing Al2O3 and SiO2 is used) lower temperatures can be used.
Coke is also formed which slows down catalytic action, so the coke must be periodically burnt away to restore catalytic activity. This provides a source of heat for the endothermic cracking reaction.
3. Isomerisation. Not only chain length is important but structure too. Isomerisation occurs when a molecule undergoes a chemical rearrangement to form a more branched isomer.
4. Catalytic reforming. A platinum catalyst is used and the process is sometimes called 'platforming'. This process converts straight-chain alkanes boiling in the petrol range into hydrocarbon rings without altering carbon number drastically, but the number of hydrogens will be reduced. This is a widely used refinery reaction as these hydrocarbons are used to replace tetraethyl lead, making unleaded fuel.
We have learnt above that, under the influence of high temperature, and particularly in the presence of certain catalysts, hydrocarbons become unstable and break or 'crack' into smaller fragments, e.g. the saturated hydrocarbon dodecane, C12H26, may be broken in the following ways:
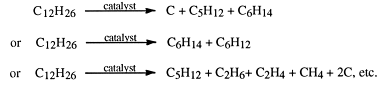
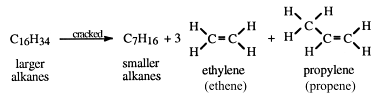
The cracking of kerosene illustrates this conversion.
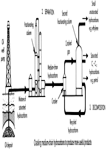
Fuels and chemicals produced
There are three basic fractions that yield petroleum. Light naphtha and heavy naphtha give mainly straight-chain alkanes - called 'straight run' petrol, which can be converted by isomerisation into petrol with a higher octane number. Some light naphtha and heavy naphtha is 'catalytically reformed' into other hydrocarbons, such as benzene, methylbenzene (toluene) and dimethyl benzene. Crude oil also contains some of these compounds. These also have a high octane rating, and can be used to replace tetraethyl lead.
Gas oil is cracked into petroleum sized molecules and these, in turn, may be subjected to isomerisation and catalytic reforming. These same three basic fractions provide six out of seven of the most important hydrocarbons in the chemical industry. Of these seven compounds, ethene, obtained by cracking, is the most important of all.
There is a competition between these compounds needed for fuel and the same compounds needed in the chemical industry. Eighty eight out of every 100 barrels of oil are used to produce energy. The other 12 barrels are used for the manufacture of a wide variety of chemicals. The petrochemical industry has grown since World War II when, because of shortages of products such as silk and rubber, substitutes were developed. After the war the research effort was redirected towards consumer goods.
The basic seven chemicals in the petrochemical industry are given in the following table:

The most important of these seven, the 'big three,' are in bold type. All of these are hydrocarbons, i.e. contain carbon and hydrogen only. Methane comes from natural gas but the remaining six are derived chemically from crude oil fractions.
The point to note is that these few compounds can be converted into many, many others - thousands of sophisticated chemicals and plastics. Organic chemicals can be interconverted quite simply using a basic set of reactions. You must realise that although many compounds are possible, the basic reactions to produce them are few. Organic chemistry is not difficult!

The simplest of the organic chemical families is a group of saturated hydrocarbons called the alkanes. All alkanes take part of their family name, the 'ane,' into their own name.
Alkanes are hydrocarbons (that is they contain carbon and hydrogen only) and are said to be saturated hydrocarbons - meaning they contain only C _ C and C _ H single bonds. They contain the maximum number of hydrogen atoms per molecule.
Crude oil also contains sulfur compounds, which are removed at the refinery. This is because if left in the petrol they would burn to
form oxides of sulfur so contributing to "acid rain". These oxides also poison the catalytic converters fitted to cars.
When an oil spill occurs it is the BTX component that does the most damage to sea birds and mammals. The sticky, tar-like blobs are difficult to remove.
Not only do we build from carbon chains, we also depend on them everyday of our lives. Foods are carbon chains, as are clothes, packaging, paper, fuels and plastics!
Can you answer the following questions?
1 Which are gases, which liquids and which solids at room temperature?
2 What is the general formula of an alkane?
The alkanes are all non-polar compounds.
What observation in the laboratory tells you that alkanes do not react with the two extremely reactive metals, sodium and potassium?
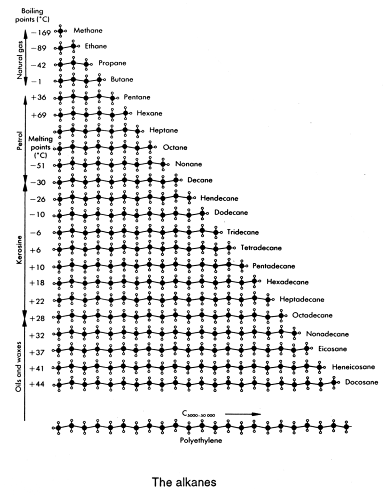
Here is a table of the first 22 alkanes - they are members of an homologous series and are all straight-chained. You can also get branches on each of these chains. What is to be appreciated is that, because of branching, the number of alkanes possible is very great. Your teacher will only expect you to know the names of the first ten alkanes.
Physical properties of alkanes
Alkanes are insoluble in water, and less dense than water. They form a layer, which floats on top. They dissolve in organic solvents, such as dichloromethane, CH2Cl2, and liquid alkanes are soluble in each other. Petrol is an example of alkanes soluble in each other.
They have low melting points and boiling points.
Chemical properties of alkanes.
They are very unreactive. Their former family name, 'the paraffins', means 'little affinity'.
1. They do not react with concentrated acids, e.g. sulfuric acid. Two non-reacting layers form. Concentrated sulfuric acid does react with most other organic compounds.
2. They do not react with concentrated alkali, e.g. concentrated sodium hydroxide solution and concentrated potassium hydroxide solution.
3. They do not react with strong oxidants.
(a) With acidified purple potassium permanganate solution there is no colour change. (A change from purple to colourless would show oxidation had occurred.)
(b) With acidified orange potassium dichromate solution there is no colour change. (A change from orange to green would show oxidation had occurred.)
Even on standing for some time, these strong oxidants show no colour change when added to any alkane.
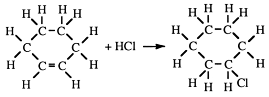
4. They do not react with bromine in the cold and in the absence of light. This is shown by no loss of colour. They do react if heated or subjected to bright light.
Substitution reactions of alkanes
A substitution reaction is one where an atom or group of atoms is displaced by another atom or group of atoms. The alkanes undergo substitution reactions with difficulty. For example:

Other substitution products may be formed:
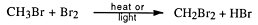

In sunlight the reaction occurs very slowly. This reaction can be used commercially to form carbon tetrachloride, an industrial solvent.
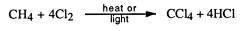
In practice, a mixture of substituted hydrocarbons is formed and these must be separated by fractional distillation. For higher alkanes, a bigger variety of products is possible.
Combustion of alkanes
From the above, it would be quite wrong to say alkanes are totally unreactive - or that they are unimportant. Commercially, they are the most important organic compounds because they occur naturally in large quantities and from them chemists make almost all other synthetic organic compounds. Their most important use is as fuels, e.g.
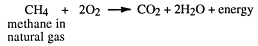
Try balancing this reaction, which occurs in the engines of motorcars:
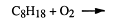
Where does the energy produced come from?
Preparation of alkanes
Because alkanes occur naturally (e.g. in natural gas), it is seldom necessary to make them in a laboratory. However, methane is prepared by bacterial decomposition of organic waste. This provides a renewable energy source. Other methods for making alkanes used in fuels are being investigated.
Microorganisms present in the dung decompose it in the absence of air to form methane gas. At sewage works, methane generated from the sewage is used to generate the electricity to power the works.
Methane is a colourless, odourless gas. A smelly impurity is added to gas used for domestic use so that gas leaks can be detected.
Jean Baptiste Dumas, a 19th century Professor of Chemistry in Paris, discovered substitution when he was asked to analyse s om e candles whic h had distressed guests at the King's reception by giving off acrid fumes. The gas was HCl and he traced this to some chlorine, which had been used to bleach the candles. He found that chlorine had reacted chemically with the candle wax, replacing some of the hydrogens with chlorines. He found bromine and iodine could replace hydrogen as well. Dumas could not gain acceptance of his ideas although they were correct and he gave up his brilliant chemical career to become a politician.
How many hydrogens can be displaced altogether in hexane? How many compounds can be formed by reacting hexane with chlorine?
It is oil that makes possible where we live, how we live, how we commute to work, how we travel.
Bacterial decomposition in the absence of air is called anaerobic.
Gas was once made from coal at a gas works. Today we use natural gas, and no longer need to make gas.
Alkenes are sometimes called olefins, meaning "oil-forming".
The type of bond in the chain, here a double bond, controls the compound's chemical properties.
Cracking is the only commercial source of ethylene (ethene) and propylene (propene).
Ethene and propene are used extensively to make plastics.
Al l al kenes are non-polar compounds.
Alkenes
The next in our family of organic compounds is named the alkenes. The general formula of this homologous series, CnH2n, shows that the alkenes have more carbon and less hydrogen in their molecules than do the alkanes.
Unsaturated hydrocarbons are those with less than the maximum amount of hydrogen. This arises because they have 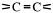
Alkenes have carbon-carbon double bonds, 
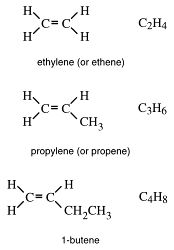
Note again that the members of this series take part of the family name.
Functional groups

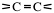
A functional group is a reactive site in a molecule or any atom or group of atoms, which cause that molecule to take part in a chemical reaction.
Each functional group has a name and 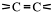
Physical properties of alkenes

Like alkanes, they have low melting points and boiling points. The first members of the homologous series are gases; again similar to the alkanes.
Chemical properties of alkenes
Alkenes are much more reactive than alkanes. This is because alkenes have a functional group. Addition and oxidation are the typical reactions.
Combustion of alkenes
Alkenes burn with luminous smoky flames. The smokiness due to unburnt carbon is typical of unsaturated hydrocarbons. With the higher carbon to hydrogen ratio, some carbon remains unburnt.
Addition of bromine
The colour of bromine rapidly disappears when bromine water is shaken with an alkene. This is used as a test for unsaturation. The two reactants form one product with a molecular formula, which is the sum of the two reactants' formulae, e.g.

The reaction site is the alkene group. The reaction is rewritten to show this:

One bromine adds to one end, the other bromine to the other. One double bond forms two new single bonds and the product is saturated.
Addition of hydrogen
e.g. 
Addition occurs to both ends as before. The hardening of vegetable oils, which are
'polyunsaturated' into margarine, is accomplished with this reaction.
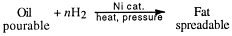
Catalytic hydrogenation continues until the fat is of the right spreading consistency.
Addition of water
An acid catalyst (usually concentrated H2SO4) is used.

This is the commercial method of making ethanol. Methylated spirits, which is about 95 per cent ethanol, is the most common industrial solvent.
Reaction with acids
Addition of HX
X can be F, Cl, Br or I (as a concentrated solution or as a gas), e.g.
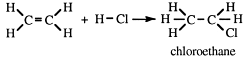
With cyclohexene the reaction would be:
Bromine, Br2, is a dark red-brown l i q u i d ; b ro mi n e w a te r i s p a l e yellow or orange, depending on concentration.
Hydrogenation can occur at room temperat ure when t he nickel catalyst is added.
A ddit ion r eac t ions ar e wher e atoms are added to the double bond in an alkene, or the triple bond in an alkyne.
Hydrogenat ion react ions also occur in living cells.
'Ant ioxidant s' are added t o polyunsaturated vegetable oils & margarine to stop them becoming rancid. The acids formed on oxidation have nauseating odours.
A polymer is a very large molecule made from simple units repeated many times.
Rubber is a polyunsaturated hydrocarbon polymer, which occurs naturally and is also made artificially. It is rapidly oxidised by ozone, a pollutant in urban areas, such as Los Angeles. Car tyres, rubber hosing, gaskets and boots are attacked.
Note that reaction only occurs at the functional group. The saturated part of the molecule, regardless of size or shape, can be ignored. Note also that these are addition reactions. Adding of atoms has occurred across the double bond.
Reaction with acidified potassium permanganate
Alkenes decolourise acidified potassium permanganate. This is another test for unsaturation but it is not as good as the bromine water test.
KMnO4/H+ is decolourised by other functional groups too. The reaction is an addition and an oxidation since oxygen adds to the alkene. On warming, the parent chain can be broken into smaller pieces due to reaction at the alkene group.
Polymer formation
Alkenes can add to each other to form alkanes, which form very large molecules called macromolecules. Their chains can contain 200 000 carbon atoms, or more! For example

Polythene is a giant alkane and the world's most common plastic. Other plastics are made from reactions similar to this.

Alkynes
A third family of hydrocarbons is called the alkynes. The general formula, CnH2n-2, shows that they too are unsaturated hydrocarbons.
The functional group, 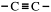
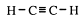
The two carbons of the functional group and the two atoms directly attached to it are in a straight line. Ethyne (acetylene) itself is a linear molecule. It is used as a heat source for joining metals by melting them together. This is called oxy-acetylene welding.
Ethyne (acetylene) is prepared in the laboratory and industry by the same reaction. Calcium dicarbide (more commonly called calcium carbide) reacts with water to produce ethyne (acetylene). The reaction is:

Calcium carbide is prepared from calcium oxide and coke at temperatures exceeding
2000°C. This makes it expensive to produce and so acetylene in turn becomes expensive. For this reason, most chemicals now being produced from ethyne (acetylene) will in future be prepared from ethene instead.
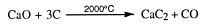
Physical properties of alkynes
The properties of all the members of the alkynes are very similar - acetylene is the example used here.
Acetylene (ethyne) is a colourless gas, insoluble in water. Other members of this homologous series have progressively higher boiling and melting points.
Chemical properties of alkynes
Combustion
1. Acetylene burns with a luminous and very smoky flame. Why is this so?
2. In pure oxygen, complete combustion occurs:
2C2H2 +5O2 → 4CO2 + 2H2O
and very high temperatures - approximately 3000°C - can be reached. This reaction is used in oxy-acetylene welding.
Addition reactions
• Ethyne (acetylene) decolourises bromine water slowly
The reaction takes place in two steps:

Addition occurs first to both ends of the triple bond, then to both ends of the double bond. Can you name the two addition products?
• Ethyne (acetylene) reacts explosively with chlorine gas
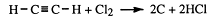
To prevent explosions, acetylene and chlorine are usually mixed in retorts with kieselguhr and iron filings to absorb the heat of the reaction. Addition compounds are then formed.
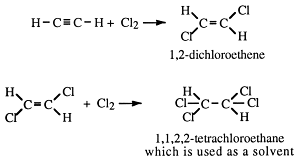
• Ethyne (acetylene) adds hydrogen
At room temperature, in the presence of a nickel catalyst, acetylene adds hydrogen. The reaction takes place in two steps. First ethene (ethylene) and then ethane are formed.
The alkynes, like the alkanes and alkenes, are all non-polar molecules.
The flame temperature of C2H2/air is 2325°C. The flame temperature of C2H2/O2 is 2975°C. The much hotter flame with oxygen is due to no nitrogen being present. N2(g) absorbs some of the heat released when acetylene burns in air.
Kieselguhr is a soft fine-grained deposit consisting of the siliceous skeletal remains of diatoms, formed in lakes and ponds. Kieselguhr is used as an absorbent filtering material, filler, and insulator.
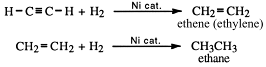
• Addition of hydrogen chloride gas
When hydrogen chloride gas is added to acetylene, chloroethene is produced - otherwise known as vinyl chloride:
Acetylene and the other members of the homologous series undergo both addition and oxidation reactions like the other family of unsaturated hydrocarbons, the alkenes.
Systematic names can be very
long indeed. F or example,
1,2,3,4,10,10-hexachloro-6,7- epoxy-1,4,4a,5,6,7,8,8a- octahydro-1,4-endo-endo-5,8- dimethanonaphthalene is the syste mati c n a me for a n
insecticide, far better known as dieldrin!
Vinyl chloride is used to produce the plastic, polyvinyl chloride or PVC.
Oxidation
Acetylene turns the purple alkaline potassium permanganate solution green. This is evidence that oxidation has occurred.

Nomenclature
Naming compounds in organic chemistry is referred to as nomenclature. Rules for naming have become necessary because there are so many compounds and their numbers are increasing.
Systematic names give the structure of the compound being referred to. Previously only unsystematic names were used which could refer to the source of the compound, e.g. jasmone from jasmine, or to a property of the compound, for example putrescine has a foul smell.
Unsystematic, 'trivial' or 'common names' abound. In fact, they are still probably used more than systematic names because of their brevity, and because when a new compound is first isolated, the structure, upon which its name is based, is frequently not known.
Lengthy cumbersome names are rarely used in day-to-day conversation, even among chemists. They are reserved for scientific publications where it is essential to give an unambiguous statement. However, the basic rules of nomenclature for simple compounds are easy to apply, and having mastered these, you will know how to convert a name to a structure and a structure to a name.
We start with the names of the alkanes because if you know the names of the first ten alkanes, you have a basis for naming hundreds of thousands of other compounds.
Parent alkanes
Learn the names of the first ten alkanes and the number of carbon atoms associated with each name.

Branches or side-chains can be short or long and these have names too. Two
important side chains are _CH3 (methyl) and _CH2CH3 (ethyl). These do not exist separately but only attached to longer carbon chains. They have one hydrogen less than the alkanes from which they are derived.
Naming branched alkanes
Names of organic compounds are frequently made up of three parts:
1. The name of the 'parent' alkane chain.
2. The name(s) of the substituent(s). (Substituent is a word that refers either to an alkyl side chain or to a functional group attached to the parent chain.)
3. The position numbers of the substituents on the parent chain.
Rules
1. Look for the longest continuous carbon chain. This is called the parent chain. e.g.
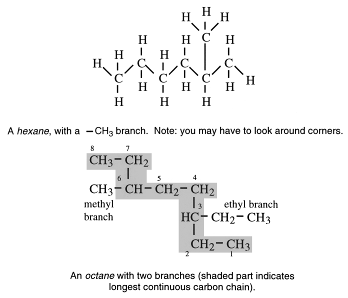
2. Number each carbon atom in sequence from the end closer to the branches.
In the example above, there is an ethyl branch on carbon atom number 3 and a methyl branch on carbon atom 6. (Each branch must have a name and a position number.)
More precisely, the above compound is a methylpentane and, since the methyl group is attached to carbon atom number two, it is 2-methylpentane. (The octane above it is 3-ethyl-6-methyloctane.)
3. Side chains are listed in alphabetical order. For example ethyl is written before methyl.
Building models is the best way of
learning about the structure of organic molecules. Borrow a model kit from your teacher.
The rule is that the substituent must be given the lowest possible number. Hence this compound is not called 4-methylpentane.
Commas separate numbers, and hyphens separate numbers and words. The final word, however long, is written as one word.
Every hydrocarbon with an alkene group has an 'ene' ending to its systematic name.
In longer alkenes and alkynes, the position number of the functional group is part of the name.
A halogen can be attached to a parent chain by a single bond like a branch. Their names become _F (fluoro), _Cl (chloro), _Br (bromo) and _I (iodo).
Remember:
1 Name the parent chain.
2 Name the substituents and put in alphabetical order.
3 Put in the position number of each substituent.
4. Two identical side chains are preceded by 'di', three by 'tri', four by 'tetra' and so on. For example:

If two side chains are on the same carbon atom, the number must appear twice. (Note: di, tri, tetra, penta and similar prefixes have no alphabetical significance.)
Exercise
According to text books there are three other isomers having the same molecular formula as the dimethylbutanes shown above. Draw graphic formulae for these compounds and name them.
Naming alkenes
Alkane → alkene
Pick the longest continuous chain containing the alkene group. This is the parent chain. The 'ane' in the parent alkane is changed to 'ene'. For example

Naming alkynes
Alkane → alkyne
Look for the longest continuous chain containing the alkyne group. This is the parent chain. The 'ane' part of the parent alkane is changed to 'yne'. What is the systematic name for acetylene?
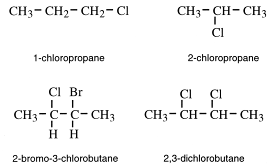
Naming haloalkanes
Source : http://www.usc.adelaide.edu.au/local/transitionlectures/chemistry/organic_chemistry.doc
Web site link: http://www.usc.adelaide.edu.au/
Google key word : Organic chemistry file type : doc
Author : not indicated on the source document of the above text
If you are the author of the text above and you not agree to share your knowledge for teaching, research, scholarship (for fair use as indicated in the United States copyrigh low) please send us an e-mail and we will remove your text quickly.
Organic chemistry
If you want to quickly find the pages about a particular topic as Organic chemistry use the following search engine:
Chemistry
Organic chemistry
Please visit our home page
Larapedia.com Terms of service and privacy page
